Insulin special packing C8I
- Product description
- 参数
Based on the series of characteristic separation materials, the process development technology of Acchrom separation and purification has been developed to cover the overall process of separation and purification, from the screening and optimization of separation materials, screening and optimization of separation conditions to the amplification and verification of separation and purification process. The Company has established a separation and purification process development and validation model from 4.6 column simulation preparation to DAC-50 column validation. From the overall improvement of drug quality, the overall upgrade of drug purification process, the removal of key drug impurities to the purification and preparation of active compounds, active analogues and drug impurity controls, the Company has formed its own technical characteristics and accumulated rich practical experience in the development of separation and purification processes in fields such as biological fermentation drugs, traditional Chinese medicine and natural products, and chemical synthesis drugs.
CASES:
1、Process amplification validation cases
2、Representative cases of drug quality improvement
3、Representative cases of pharmaceutical purification processes
4、Cases of natural product compound preparation
5、Cases of biofermentation slimming drug isolation and purification
Introduction to Packing
- The surface bonding group is mainly C8
- Exclusive bonding density regulation, suitable for the separation of insulin-like peptide samples
- Deep end-capping technology, low silanol group residue, excellent peak shape for alkaline samples
- Excellent stability under alkaline conditions, extended service life
- Product specifications are as follows:
| Name | Grain size μm | Pore size Å | Carbon content % | pH range of application |
| C8I | 10 | 100 | 11 | 1~12 |
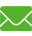
Purification of recombinant human insulin
Simulated preparation
| Retention time min | Purity % | Yield % | Macromolecular protein impurities % | |
| Injection 1 | 74.89 | 98.70 | 94.11 | 0.19 |
| Injection 2 | 75.76 | 99.45 | 93.47 | 0.20 |
| Injection 3 | 76.28 | 99.55 | 94.02 | 0.22 |
Chromatographic column: C8I (4.6mmx 250mm) ;
Flow rate: 0.5mL/min ;Sample loading capacity: 2% ;
Elution: Buffered salt-acetonitrile system, with a relatively wide range of application
Purification of glargine insulin
Simulated preparation

Chromatographic column: C8I (4.6mmx 250mm) ;
Flow rate: 0.5mL/min ;Sample loading capacity: 1.3% ;
Elution: Buffered salt-acetonitrile system, with a relatively wide range of application

| Purity of original sample % | Purity after purification % | Yield % |
| 84.92 | 99.05 | 93.88 |
Chromatographic column: C8I (30mm*250mm, 100g packing) ;
Flow rate: 18 mL/min;Sample loading capacity: 1.5% ;
Elution: Buffered salt-acetonitrile system, with a relatively wide range of application
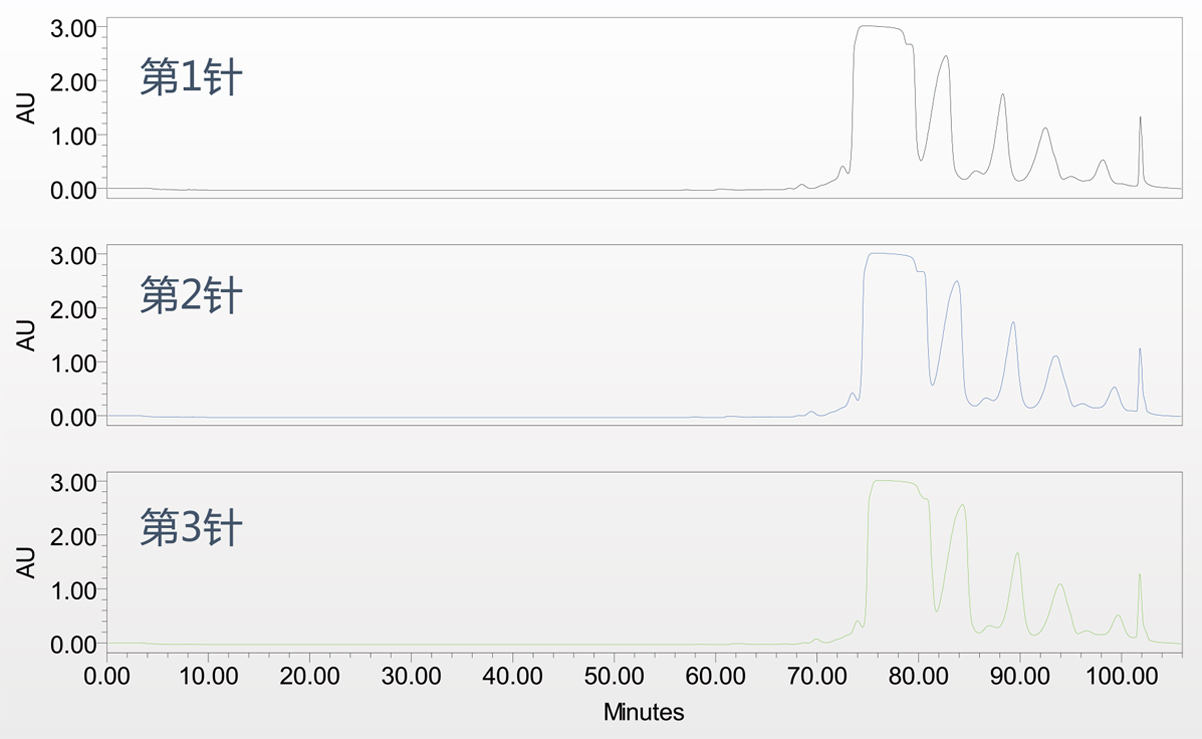
Production application( 800 DAC )

| Name of packing | Product purity | Recovery rate |
| An imported K brand | 99.45% | 72% |
| Acchrom C8I | 99.58% | 75% |
★Purification results of glargine insulin from a domestic biopharmaceutical company (5 batches of packing have been accomplished)
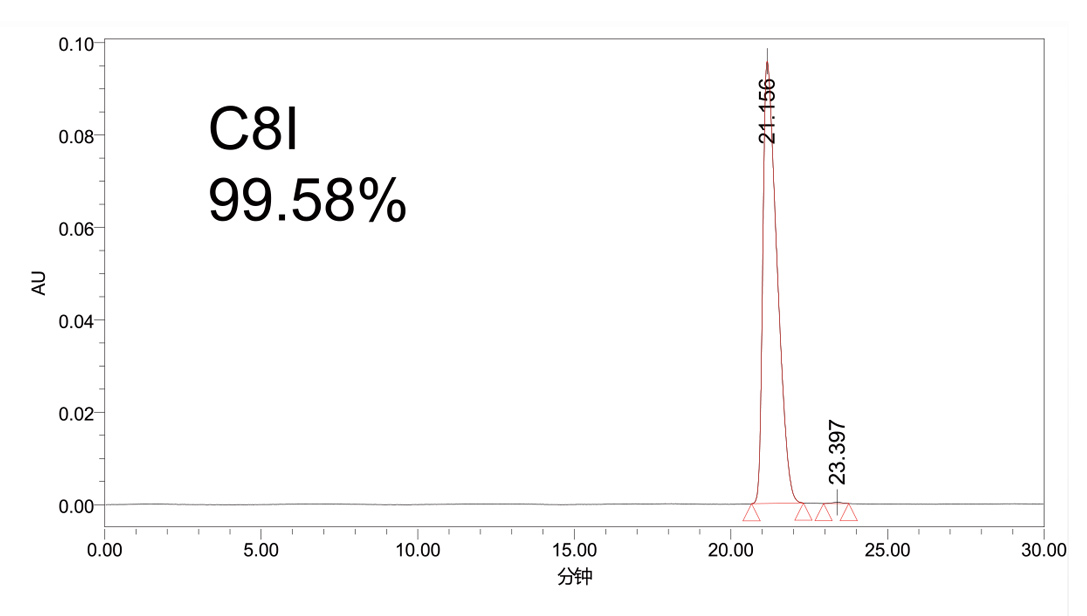
Alkali resistance test
Column efficiency and retention changes
 Note: The strongly adsorbed heteroproteins in insulin samples are prone to contaminate the packing material and hence affect the separation. Strong alkaline solution is required to regenerate the packing after a period of operation. The silica matrix packing features poor stability under strong alkaline conditions, making alkaline resistance an important factor in determining the life span of insulin packing. Test conditions: 0.1M NaOH/EtOH =50/50 Rinse 30 min, neutralize with 0.2 M HAc, test column efficiency and pressure, then cycle the test.
Note: The strongly adsorbed heteroproteins in insulin samples are prone to contaminate the packing material and hence affect the separation. Strong alkaline solution is required to regenerate the packing after a period of operation. The silica matrix packing features poor stability under strong alkaline conditions, making alkaline resistance an important factor in determining the life span of insulin packing. Test conditions: 0.1M NaOH/EtOH =50/50 Rinse 30 min, neutralize with 0.2 M HAc, test column efficiency and pressure, then cycle the test.
★C8I has excellent alkaline tolerance, with chemical stability comparable to or even slightly superior to that of an imported K brand packing.
Contact us
Add:No.1 Bingang Road, Jingang Development Zone, Binhai Town, Wenling, Zhejiang Province
E-mial:marketing@acchrom.com
Tel:4009653365
Phone:(East)13326073588